A solvent-free, base-catalyzed domino reaction towards trifluoromethylated benzenes from bio-based methyl coumalate†
Abstract
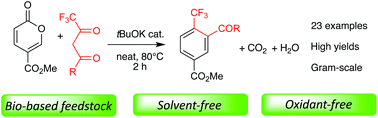
A novel, efficient, and environmentally compatible method for CF3-substituted benzene production is reported. It sources a bio-based feedstock, employs tBuOK as a catalyst, and is solvent-free. This regioselective approach provides various trifluoromethyl benzenes in good to excellent yields, without any extra oxidant or special care. CO2 and water are the only byproducts of this process, and the reaction conditions can scale up to gram quantities. The transformation involves an unprecedented tBuOK-catalyzed domino process, and features Michael addition/6π-electrocyclic ring opening/[1,5]-H shift/carba-6π-electrocyclic ring closure/decarboxylative aromatization reactions.


 Please wait while we load your content...
Please wait while we load your content...