Alteration of the structure of rice proteins by their interaction with soy protein isolates to design novel protein composites
Abstract
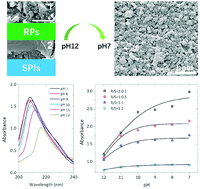
Rice proteins (RPs) are plant proteins with high nutritional value, but they attract little industrial interest because they are poorly soluble (<2%, w/v), with poor solubility-related functionalities. We report that by mixing RPs and soy protein isolates (SPIs) in RP/SPI ratios of 1 : 0.1–1 : 2 at pH 12, and then neutralizing the solution to pH 7 at ambient temperature, the solubility of RPs can be boosted to over 80% without any damage to the subunits of either protein. Structural and morphological characterization showed that the RPs and SPIs interacted at pH 12 and formed complexed structures that underwent dramatic conformational changes during neutralization, folding together into nanoscale spheres with high surface charges (zeta potential >30 mV). With their enhanced solubility and retained hydrophobicity, the protein composites displayed excellent interfacial affinities. The emulsifying and foaming capacities and stabilities of the protein composites were almost doubled or even tripled at pH 7 relative to those of RPs. Furthermore, the protein composites maintained the balanced amino acid compositions of both the RPs and SPIs. Our results demonstrate that inducing protein interactions by modulating the pH is a robust technique for the functional and nutritional modification of food proteins.


 Please wait while we load your content...
Please wait while we load your content...