Quantum and classical IR spectra of (HCOOH)2, (DCOOH)2 and (DCOOD)2 using ab initio potential energy and dipole moment surfaces
Abstract
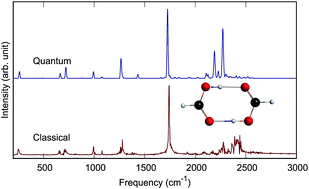
An accurate quantum mechanical description of the vibrational dynamics and IR spectra of molecules is illustrated here for the formic acid dimer, (HCOOH)2 and its isotopologues (DCOOH)2 and (DCOOD)2 in full dimensionality. The calculations make use of recent full-dimensional ab initio potential energy and dipole moment surfaces and are done with the code MULTIMODE. IR spectra are reported for the three dimers and also compared to available experimental spectra. In addition, standard classical and “semiclassically prepared” quasiclassical molecular dynamics calculations of the IR spectra of these complexes are reported and compared to the quantum spectra and also experiment. These comparisons indicate good accuracy of the MD spectra for sharp bands but not for the complex O–H stretch band, where the complex molecular dynamics band is upshifted from experiments by roughly 300 cm−1. For the fully deuterated dimer (DCOOD)2, the quantum spectral band for the O–D stretch sharpens relative to the O–H spectral bands in (HCOOH)2 and (DCOOH)2; however, the molecular dynamics OD stretch band does not exhibit this sharpening.
- This article is part of the themed collection: Quantum effects in small molecular systems


 Please wait while we load your content...
Please wait while we load your content...
