Materials discovery by chemical analogy: role of oxidation states in structure prediction†
Abstract
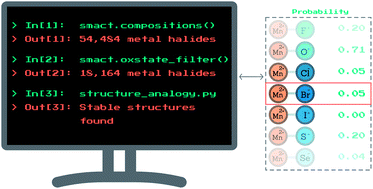
The likelihood of an element to adopt a specific oxidation state in a solid, given a certain set of neighbours, might often be obvious to a trained chemist. However, encoding this information for use in high-throughput searches presents a significant challenge. We carry out a statistical analysis of the occurrence of oxidation states in 16 735 ordered, inorganic compounds and show that a large number of cations are only likely to exhibit certain oxidation states in combination with particular anions. We use this data to build a model that ascribes probabilities to the formation of hypothetical compounds, given the proposed oxidation states of their constituent species. The model is then used as part of a high-throughput materials design process, which significantly narrows down the vast compositional search space for new ternary metal halide compounds. Finally, we employ a machine learning analysis of existing compounds to suggest likely structures for a small subset of the candidate compositions. We predict two new compounds, MnZnBr4 and YSnF7, that are thermodynamically stable according to density functional theory, as well as four compounds, MnCdBr4, MnRu2Br8, ScZnF5 and ZnCoBr4, which lie within the window of metastability.
- This article is part of the themed collection: Methods and applications of crystal structure prediction


 Please wait while we load your content...
Please wait while we load your content...