Immobilization of hydrous iron oxides in porous alginate beads for arsenic removal from water†
Abstract
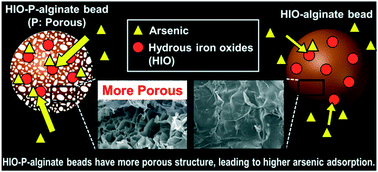
For removal of arsenic in the aqueous phase, hydrous iron oxides (HIOs) were immobilized in alginate beads with enhanced porosity (designated as HIO-P-alginate beads). The HIO-P-alginate beads had macropores, observed by SEM, as well as mesopores and featured a higher BET surface area than previously developed adsorbent beads. Thus, the adsorption of As(III) and As(V) by the HIO-P-alginate beads was more rapid than that of previously reported HIO-alginate adsorbents. The kinetics of adsorption were well described by a pseudo-second-order model, indicating that chemisorption mainly governed the As(III) and As(V) adsorption. We confirmed a chemisorption mechanism for the As(III) and As(V) adsorption, through isotherm studies using the Dubinin–Radushkevich isotherm model. The application of an intraparticle diffusion model to the kinetic data suggested that the As(V) adsorption onto the HIO-P-alginate beads was controlled entirely by intraparticle diffusion whereas the As(III) adsorption was governed by intraparticle diffusion only at short contact times. As(III) adsorption was highest at neutral pH; however, As(V) adsorption was highest at low pH. Both As(III) and As(V) adsorption did not compete with nitrate adsorption, and the As adsorption improved with increasing ionic strength. The HIO-P-alginate beads could be regenerated several times with a NaOH solution and were successfully reused for arsenic removal.


 Please wait while we load your content...
Please wait while we load your content...