1,3,5-Trimethoxybenzene (TMB) as a new quencher for preserving redox-labile disinfection byproducts and for quantifying free chlorine and free bromine†
Abstract
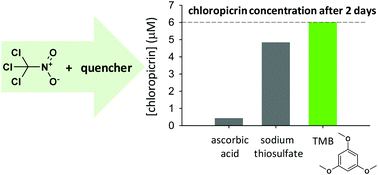
Sodium sulfite, sodium thiosulfate, and ascorbic acid are commonly used to quench free chlorine and free bromine in studies of disinfection byproducts (DBPs) in drinking water, wastewater, and recreational water. The reducing capabilities of these quenchers, however, can lead to the degradation of some redox-labile analytes. Ammonium chloride, another common quencher, converts free chlorine into monochloramine and is therefore inappropriate for analytes susceptible to chloramination. Herein, we demonstrate the utility of 1,3,5-trimethoxybenzene (TMB) as a quencher of free chlorine and free bromine. The reactivity of TMB toward free chlorine was characterized previously. The reactivity of TMB toward free bromine was quantified herein (kHOBr,TMB = 3.35 × 106 M−1 s−1) using competition kinetics. To explore the feasibility of TMB serving as a free halogen quencher for kinetic experiments, chlorination of 2,4-dichlorophenol, bromination of anisole, and chlorination and bromination of dimethenamid-P were examined. Although TMB does not react with free chlorine or free bromine as quickly as do some (but not all) traditional quenchers, there was generally no significant difference in the experimental rate constants with TMB (relative to thiosulfate) as the quencher. By monitoring the chlorination and bromination products of TMB, free halogen residuals in quenched samples were quantified. Furthermore, TMB did not affect the stabilities of DBPs (e.g., chloropicrin and bromoacetonitriles) that otherwise degraded in the presence of traditional quenchers. TMB could, therefore, be an appropriate quencher of free chlorine and free bromine in aqueous halogenation experiments involving redox-labile analytes and/or when selective quantification of residual free halogens is desired.
- This article is part of the themed collection: Best Papers 2018 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...