Retracted Article: Influence of aqueous sulfide on speciation of U(vi) adsorbed to nanomagnetite†
Abstract
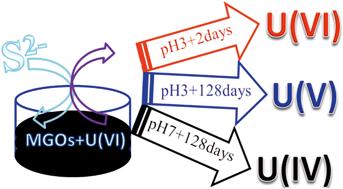
Heterogeneous reduction of U(VI) by structural Fe2+ and S2− is a key process influencing its fate and transport in subsurface environment. In this study, the adsorption of U(VI) on nanomagnetite was primarily conducted and then, it was desorbed by adding S2−. The desorption of absorbed U(VI) significantly increased with the increase in S2−, whereas S2− consumption was higher than the desorbed amount of U(VI) due to the volatilization (H2S) and sulfidization (FeS) at low and high pH, respectively. The XRD analysis demonstrated that the main components of corrosion products for nanomagnetite after adding S2− at pH 3.0 and 7.0 are lepidocrocite and mackinawite, respectively, after a reaction time of 128 days. According to analyses of ITFA and XANES spectra, the main speciation of uranium at pH 3.0 and 7.0 were U(V) and U(IV) species, respectively. The required energy of U(V) desorbed from lepidocrocite (−12.78 kcal mol−1) at pH 3.0 after 128 days was higher than that of U(IV) (−26.16 kcal mol−1) by theoretical calculations, indicating that U(V) can be stably incorporated into the secondary phase. These findings are crucial for understanding the speciation of uranium at low pH and reducing conditions.


 Please wait while we load your content...
Please wait while we load your content...