Modeling coupled kinetics of antimony adsorption/desorption and oxidation on manganese oxides†
Abstract
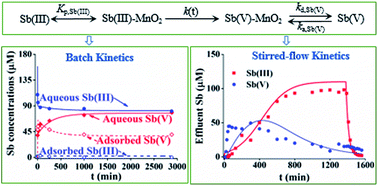
Understanding the kinetic reactions of antimony (Sb) at the MnO2–water interface is essential for predicting the dynamic behavior of Sb in soil environments. In this study, we developed a quantitative model for assessing the coupling between Sb(III) oxidation and Sb(III)/Sb(V) adsorption/desorption kinetics and the role of each individual reaction process in controlling the overall reaction rates. Based on our modeling results, Sb(III) oxidation rates were very fast at the early stages of the reactions and may significantly slow down with time, and Sb(III) and Sb(V) showed different adsorption behavior on δ-MnO2. Our kinetics model is able to account for different adsorption/desorption kinetics of Sb(III) and Sb(V) and the changes of Sb(III) oxidation rates during the coupled kinetic processes, which provides a general modeling framework for predicting Sb kinetic behavior at the MnO2–water interface.


 Please wait while we load your content...
Please wait while we load your content...