Effects of calcium on the kinetics of a model disjunctive ligand exchange reaction: implications for dynamic trace metal ion speciation†
Abstract
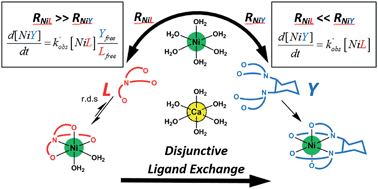
Trace metal ion speciation in natural waters is often under kinetic control due to slow exchange reactions involving multidentate ligands (both natural and anthropogenic) and constituent ions (e.g. calcium). Incomplete understanding of the kinetic behavior (rates, rate laws, and mechanisms) of multidentate ligand exchange reactions hinders prediction of metal ion bioavailability and mobility in these systems. Here, we aim to improve understanding (1) by examining the mechanism by which calcium suppresses ligand exchange rates and (2) by developing conceptual tools for predicting the kinetic behavior in environmental systems. Using capillary electrophoresis, we examined the effect of calcium concentration on the kinetics of a model disjunctive multidentate ligand exchange reaction between nickel(II)-nitrilotriacetate (NiNTA) and trans-1,2-cyclohexylenedinitrilotetraacetate (CDTA) at constant temperature (25 °C) and ionic strength (10 mM). Initial NiCDTA formation rates under varying reactant and calcium concentrations were fit with a single disjunctive ligand exchange kinetic model. While the overall reaction pathway is disjunctive under all conditions studied, the presence of calcium causes a non-linear decrease in reaction rates and alters the overall reaction order with respect to free multidentate ligand concentration. The overall rate law is affected by the relative calcium affinity of exchanging ligands, which controls the balance of product formation and reactant reformation rates from the free metal ion intermediate. The log ratio of these rates is proposed as a metric for determining the appropriate simplified form of the rate law for disjunctive ligand exchange reactions. The implications for in situ ligand lability assays and metal ion bioavailability are discussed.


 Please wait while we load your content...
Please wait while we load your content...