Nanostructured multi-block copolymer single-ion conductors for safer high-performance lithium batteries†
Abstract
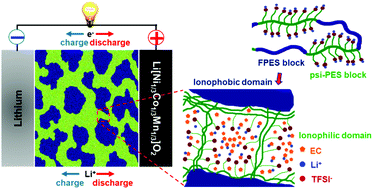
The greatest challenges towards the worldwide success of battery-powered electric vehicles revolve around the safety and energy density of the battery. Single-ion conducting polymer electrolytes address both challenges by replacing the flammable and unstable liquid electrolytes and enabling dendrite-free cycling of high-energy lithium metal anodes. To date, however, their commercial use has been hindered by insufficient ionic conductivities at ambient temperature (commonly not exceeding 10−6 S cm−1) and the limited electrochemical stability towards oxidation, in particular when incorporating ether-type building blocks, limiting their application to rather low-voltage cathode materials like LiFePO4. Here, we introduce ether-free, nanostructured multi-block copolymers as single-ion conducting electrolytes, providing high thermal stability and self-extinguishing properties and, if plasticized with ethylene carbonate, ionic conductivities exceeding 10−3 S cm−1 above 30 °C, i.e., approaching that of state-of-the-art liquid electrolytes. Moreover, these single-ion conducting ionomers present highly reversible lithium cycling for more than 1000 h and, as a result of their excellent electrochemical stability, highly stable cycling of Li[Ni1/3Co1/3Mn1/3]O2 cathodes. To the best of our knowledge, this is the first polymer electrolyte that presents such remarkable ionic conductivity and outstanding electrochemical stability towards both reduction and oxidation, thus, paving the way for advanced high-energy lithium metal batteries. Remarkably, the realization of well-defined continuous ionic domains appears to be the key to efficient charge transport through the electrolyte bulk and across the electrode/electrolyte interface, highlighting the importance of the self-assembling nanostructure. The latter is achieved by carefully (i) designing the copolymer structure, i.e., introducing alternating ionic blocks with a very regular distribution of weakly coordinating anions along the polymer chain and rigid blocks, which are completely immiscible with ethylene carbonate, and (ii) choosing the processing solvent, taking into account its interaction with the different copolymer blocks.


 Please wait while we load your content...
Please wait while we load your content...