Carbon dioxide splitting using an electro-thermochemical hybrid looping strategy†
Abstract
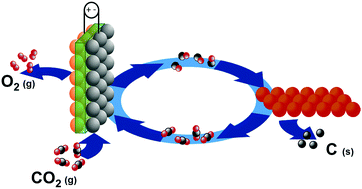
Reclaiming oxygen (O2) efficiently from carbon dioxide (CO2), a major product of human metabolism, is a key technology to minimize the oxygen supply required for challenging missions such as manned deep space exploration. Ideally, a method that can convert CO2 into elemental carbon (C) and O2 under mild conditions is highly desirable; however, due to the highly unfavorable thermodynamics, direct CO2 splitting requires extreme conditions. Herein, we present an electro-thermochemical hybrid looping (ETHL) strategy to split CO2 into C and O2 under mild conditions with a 100% theoretical oxygen recovery efficiency, which cannot be accomplished using any existing electrochemical or thermochemical process. The ETHL process combines an electrochemical step that reduces CO2 into carbon monoxide (CO) with a thermochemical step that further converts CO into C and CO2. By recycling the produced CO2 from the thermochemical step back to the first electrochemical step, this closes the loop with C and O2 as the only products. The results showed 96% selectivity for the CO2 electrolysis step and nearly 100% selectivity for the CO thermochemical step, demonstrating the feasibility of the ETHL strategy for complete oxygen recovery from CO2.


 Please wait while we load your content...
Please wait while we load your content...