Tuning mobility and stability of lithium ion conductors based on lattice dynamics†
Abstract
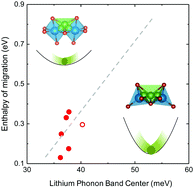
Lithium ion conductivity in many structural families can be tuned by many orders of magnitude, with some rivaling that of liquid electrolytes at room temperature. Unfortunately, fast lithium conductors exhibit poor stability against lithium battery electrodes. In this article, we report a fundamentally new approach to alter ion mobility and stability against oxidation of lithium ion conductors using lattice dynamics. By combining inelastic neutron scattering measurements with density functional theory, fast lithium conductors were shown to have low lithium vibration frequency or low center of lithium phonon density of states. On the other hand, lowering anion phonon densities of states reduces the stability against electrochemical oxidation. Olivines with low lithium band centers but high anion band centers are promising lithium ion conductors with high ion conductivity and stability. Such findings highlight new strategies in controlling lattice dynamics to discover new lithium ion conductors with enhanced conductivity and stability.
- This article is part of the themed collections: 2018 Energy and Environmental Science HOT Articles and Celebrating Excellence in Research: 100 Women of Chemistry


 Please wait while we load your content...
Please wait while we load your content...