Ligand exchange processes in zirconocene dichloride–trimethylaluminum bimetallic systems and their catalytic properties in reaction with alkenes†
Abstract
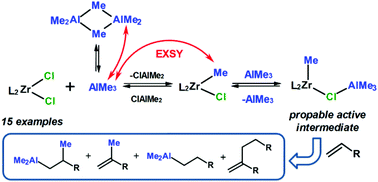
Ligand exchange processes in the systems L2ZrCl2–AlMe3 (L2ZrCl2: Cp2ZrCl2, (CpMe)2ZrCl2, (C5Me5)2ZrCl2, Me2SiCp2ZrCl2, Me2Si(C5Me4)2ZrCl2, rac-Me2C(2-Me-4-But-Cp)2ZrCl2, meso-Me2C(2-Me-4-But-Cp)2ZrCl2, rac-Me2C(3-But-Cp)2ZrCl2, Ind2ZrCl2, rac-H2C(Ind)2ZrCl2, rac-Me2C(Ind)2ZrCl2, rac-Me2Si(Ind)2ZrCl2, rac-C2H4(Ind)2ZrCl2, rac-C2H4(THInd)2ZrCl2, rac-Me2Si(THInd)2ZrCl2) and Cp2ZrMeCl2−n–AlMe3 (n = 1, 2) were studied by NMR spectroscopy with the goal to establish the structures and dynamic features of probable intermediates in the zirconocene-catalyzed reactions of alkenes with AlMe3. The effect of solvent, the organoaluminum compound concentration and the addition of (ClAlMe2)2 on the activation parameters of the alkyl exchange in the trimethylaluminum dimer was studied as well. The constants and activation parameters of the methyl group exchange in the monoalkyl-substituted ansa-complexes L2ZrMeCl (L2 = rac-Me2C(2-Me-4-But-Cp)2, rac-Me2C(3-But-Cp)2, rac-H2CInd2, rac-Me2CInd2, rac-Me2SiInd2, rac-H4C2Ind2) were established for the first time. The catalytic activity and chemoselectivity of zirconocenes in the reaction of alkenes with AlMe3 were evaluated and compared with the exchange and equilibrium constants of ligand exchange processes. A mechanism of the reaction was proposed.


 Please wait while we load your content...
Please wait while we load your content...