Multiple coordination modes of a new ditopic bis(pyrazolyl)methane-based ligand†
Abstract
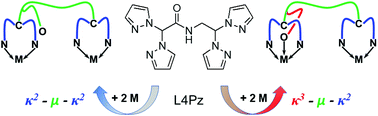
A new ditopic ligand, N-(2,2-bis(pyrazolyl)ethyl)-2,2-bis(pyrazolyl)acetamide ((pz)2CH–C(O)–NH–CH2–CH(pz)2, L4Pz, pz = pyrazolyl ring), comprising two bis(pyrazolyl)methane donor groups linked via an amide bridge, has been prepared from the reaction of HOOCCH(pz)2 and H2NCH2CH(pz)2. The ligand coordinates to various metallic salts (i.e. AgO3SCF3, PdCl2, Re(CO)5Br, and Fe(BF4)2), in either a κ2–μ–κ2 or a κ3–μ–κ2 fashion, depending on the coordination preferences of the metallic center. These compounds were characterized by NMR, UV-Vis and IR spectroscopy, and in solid state by single crystal X-ray diffraction. In the case of silver(I), a mono-dimensional coordination polymer was obtained, while the others were found to be discrete complexes. The synthesis and characterization of a heterobimetallic complex is also described. In solid state, all compounds are associated into supramolecular architectures via hydrogen bonding and pyrazolyl embrace interactions.


 Please wait while we load your content...
Please wait while we load your content...
