Evidence of proton-coupled mixed-valency by electrochemical behavior on transition metal complex dimers bridged by two Ag+ ions†
Abstract
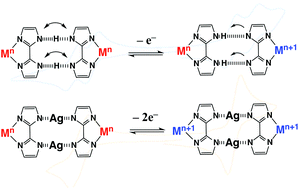
H-Bonded metal complex dimers with reversible redox behaviour, which are connected by a low-barrier hydrogen bond (LBHB) with a very low energy barrier for proton transfer, can provide a unique mixed-valency state stabilized by the proton-coupled electron transfer (PCET) phenomenon. Using cyclic voltammetry measurements, newly prepared [ReIIICl2(PnPr3)2(Hbim)]2 (2) and [OsIIICl2(PnPr3)2(Hbim)]2 (3) existing as H-bonded dimers in a CH2Cl2 solution showed a four-step and four-electron transfer containing two mixed-valency states of ReIIReIII and ReIIIReIV, and OsIIOsIII and OsIIIOsVI, respectively. Furthermore, [ReIIICl2(PnPr3)2(Agbim)]2 (4) and [OsIIICl2(PnPr3)2(Agbim)]2 (5), bridged by two Ag+ ions instead of two H-bonding protons, were prepared, and their electrochemical behaviours changed to a two-step and four-electron transfer. It is clear that the H-bonded complex dimers 2 and 3, connected by an LBHB, can be electrochemically stabilized into unique pairs of mixed-valency states by PCET, and the H-bonding proton transfer also controls the electrochemical redox behaviour.


 Please wait while we load your content...
Please wait while we load your content...