Thermal dehydrochlorination in the 4-fluoroaniline–trichloroborane system: identification of reactive intermediates involved in the formation of B,B′,B′′-trichloro-N,N′,N′′-tri((4-fluoro)phenyl)borazine†
Abstract
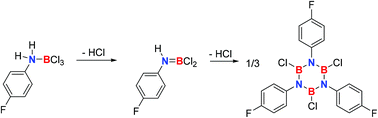
Borazines are used in chemical vapor deposition processes to produce hybrid graphene–boron nitride nanostructures. As the knowledge on the mechanism of borazine formation is scarce, we studied the mechanism of formation of B,B′,B′′-trichloro-N,N′,N′′-tri(p-fluorophenyl)borazine (3a) from p-fluoroaniline and boron trichloride employing NMR spectroscopy, X-ray single crystal structure analysis, trapping experiments, and computational chemistry methods up to the coupled cluster CCSD(T) level of theory. These studies suggest the initial formation of the 1 : 1 adduct 1a (ArNH2BCl3, Ar = 4-fluorophenyl) with a dative B–N bond that could be fully characterized including single crystal X-ray diffraction. Adduct 1a undergoes unimolecular hydrogen chloride elimination with a first-order rate constant of k1 = 3.03(7) × 10−2 min−1 in toluene at 100 °C. This rate constant is in very good agreement with the one derived (k1 = 3.18 × 10−2 min−1) from computed activation parameters (ΔH‡373.15 = 28.1 kcal mol−1, ΔS‡373.15 = 1.56 eu, ΔG‡373.15 = 27.6 kcal mol−1). The product of the first hydrogen chloride evolution is anilinodichloroborane ArNHBCl2 (2a). Compound 2a cannot be isolated in a pure form due to instability, but its presence as a transient reactive intermediate can be derived from NMR spectroscopy. Reactive intermediates other than anilinodichloroborane cannot be assigned by NMR spectroscopy. We propose that the mechanism of formation of borazine 3a involves the reaction of 2a with 4-fluoroaniline as the rate determining step.


 Please wait while we load your content...
Please wait while we load your content...