Remarkably improved electrochemical hydrogen storage by multi-walled carbon nanotubes decorated with nanoporous bimetallic Fe–Ag/TiO2 nanoparticles
Abstract
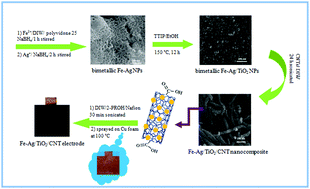
Nanoporous bimetallic Fe–Ag nanoparticles (NPs) were synthesized using a facile chemical reduction method and used to decorate the surface of multi-walled carbon nanotubes (MWCNTs) for hydrogen sorption and storage. The effect of TiO2 nanoparticles on the hydrogen storage properties of Fe–Ag/CNTs was further studied in detail. For this purpose, several nanocomposites of nanoporous bimetallic Fe–Ag/TiO2 nanoparticles with different amounts of bimetallic Fe–Ag NPs were prepared via a hydrothermal method. The hydrogen storage capacity of the as-prepared nanocomposites was studied using electrochemical methods. The Fe–Ag/TiO2/CNT nanocomposite with 0.04 M bimetallic Fe–Ag NPs showed the highest capacity for hydrogen storage, which was ∼5× higher than that of pristine MWCNTs. The maximum discharge capacity was 2931 mA h g−1, corresponding to a 10.94 wt% hydrogen storage capacity. Furthermore, a 379% increase in discharge capacity was measured after 20 cycles. These results show that Fe–Ag/TiO2/CNT electrodes display superior cycling stability and high reversible capacity, which is attractive for battery applications.


 Please wait while we load your content...
Please wait while we load your content...
