Single molecule magnet behaviors of Zn4Ln2 (Ln = DyIII, TbIII) complexes with multidentate organic ligands formed by absorption of CO2 in air through in situ reactions†
Abstract
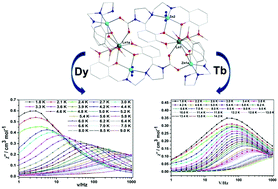
In this work, we report the syntheses, crystal structures and magnetic properties of three novel Zn–Ln mixed metal complexes, namely [Zn4Dy2(L1)2(L2)2(N3)2]Cl2·2H2O (1), [Zn4Tb2(L1)2(L2)2(Cl)2][ZnN3Cl3]·2H2O (2), and [Zn4Gd2(L1)2(L2)2(Cl)2][ZnN3Cl3]·2H2O (3), in which L12− and L23− were formed from the ligand L [L = N1,N3-bis(3-methoxysalicylidene)diethylenetriamine] through in situ reactions. Interestingly, carbon dioxide in air was absorbed in the process of forming carbamate ligand L23−; this can be ascribed to the insertion of CO2 into M–N amide bonds. Moreover, 1 and 2 represent the first series of 3d–4f SMMs containing carbamate ligands by fixation of CO2 in air. Single-crystal X-ray diffraction analyses reveal that the crystal structures of 1 and 2 are anion-dependent, i.e., the apical positions of the two ZnII ions in 1 and 2 are occupied by an N atom of N3− and by Cl−, respectively. However, the topologies of 2 and 3 are similar. Two ZnII ions and one LnIII (Ln = Dy (1), Tb (2) and Gd (3)) form nearly linear trinuclear [Zn2Ln] units which are double-bridged by two L23− ligands. Magnetic studies reveal that two complexes show single molecule magnet behavior under a direct current (dc) field, with effective energy barriers (Ueff) of 30.66(5) K for 1 and 8.87(3) K for 2. Ab initio calculations reveal that the DyIII ions in 1 and the TbIII ions in 2 are axial in nature; however, a difference in the tunnel splitting of 1 and 2 leads to variation in the magnetization blockades of the two complexes. Theoretical calculations also indicate that the directions of the main magnetic axes severely deviate from the coordination atoms of the first spheres of DyIII and TbIII in 1 and 2; thus further results in poor SMM behavior of the two complexes.


 Please wait while we load your content...
Please wait while we load your content...