Tuning the photophysical properties of carboranyl luminophores by closo- to nido-carborane conversion and application to OFF–ON fluoride sensing†
Abstract
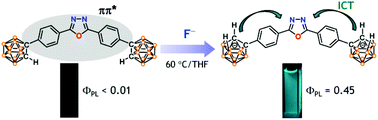
A family of closo-carborane-appended luminophores (closo-OXD1–2 and closo-DPS1–2) in which 2-R-o-carboranes (R = H, Me) are attached to the diphenyl-1,3,4-oxadiazole (OXD) or diphenyl-sulfone (DPS) acceptor groups were prepared and characterized. Deboronation of the closo-carborane cage produced the corresponding nido-carboranyl luminophores (nido-OXD1–2 and nido-DPS1–2). Whereas the closo-compounds were poorly emissive in THF (ΦPL < 0.01), the nido-luminophores exhibited an intense fluorescence with good quantum yields (ΦPL = 0.1–0.45). Electrochemical studies showed that while the closo-OXD and -DPS compounds displayed only carborane-centred, quasi-reversible reduction, the nido-compounds exhibited the typical features for nido-carborane-centred, irreversible oxidation and acceptor-centred, reversible reduction. Theoretical studies suggested that while the 1ππ* state of closo-compounds is nonemissive due to the contribution of closo-carborane to the LUMO in the S1 excited state, the intramolecular charge transfer (ICT) state from the nido-carborane to acceptor moieties in nido-compounds leads to an efficient fluorescence. Finally, THF solutions of closo-OXD1 and -DPS1 showed strong fluorescence upon the addition of fluoride anions under mild heating, but were intact to other anions, including cyanide, allowing the selective OFF–ON fluorescence sensing of fluoride.


 Please wait while we load your content...
Please wait while we load your content...