Catalytic and stoichiometric flavanone oxidation mediated by nonheme oxoiron(iv) complexes as flavone synthase mimics: kinetic, mechanistic and computational studies†
Abstract
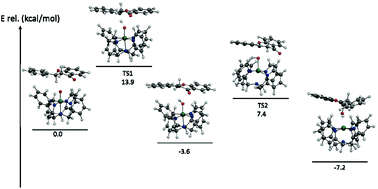
The present study describes the first example of the stoichiometric and catalytic oxidation of flavanone by synthetic nonheme oxoiron(IV) complexes and their precursor iron(II) complexes with m-CPBA as the terminal oxidant. These models, including detailed kinetic, mechanistic and computational studies, may serve as the biomimics of flavone synthase (FS) enzymes.


 Please wait while we load your content...
Please wait while we load your content...