Polynuclear Cu(i) and Ag(i) phosphine complexes containing multi-dentate polytopic ligands: syntheses, crystal structures and photoluminescence properties†
Abstract
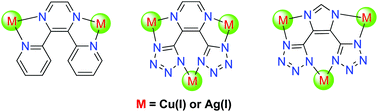
A series of polynuclear metal complexes, [Cu2(L1)(PPh3)4](ClO4)2 (1), [Cu3(L2)(PPh3)6](ClO4) (2), [Cu3(L3)(PPh3)6] (3), [Ag2(L1)(PPh3)4](BF4)2 (4), [Ag4(L2)2(PPh3)6] (5) and [Ag3(L3)(PPh3)5] (6), have been obtained from the reactions of the highly conjugated bridging ligands 2,3-bis(2-pyridyl)pyrazine (L1), 2,3-bis(2-tetrazoyl)pyrazine (H2L2) and 2,3-bis(2-tetrazoyl)imidazole (H3L3) with [Cu(MeCN)4]ClO4 and AgBF4, respectively. Their crystal structures have been determined by X-ray crystallography and their photophysical properties have been investigated in detail. Complexes 1 and 3 show photoluminescence in CH2Cl2 solution, while all the complexes exhibit obvious luminescence in the solid state; detailed photophysical studies and density functional theory calculations of these complexes have revealed that their lowest energy absorptions and emissions are predominantly derived from either metal-to-ligand charge-transfer (MLCT) or intraligand (IL) excited states.


 Please wait while we load your content...
Please wait while we load your content...