A cyanide-bridged di-manganese carbonyl complex that photochemically reduces CO2 to CO†
Abstract
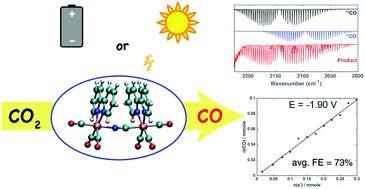
Manganese(I) tricarbonyl complexes such as [Mn(bpy)(CO)3L] (L = Br, or CN) are known to be electrocatalysts for CO2 reduction to CO. However, due to their rapid photodegradation under UV and visible light, these monomeric manganese complexes have not been considered as photocatalysts for CO2 reduction without the use of a photosensitizer. In this paper, we report a cyanide-bridged di-manganese complex, {[Mn(bpy)(CO)3]2(μ-CN)}ClO4, which is both electrocatalytic and photochemically active for CO2 reduction to CO. Compared to the [Mn(bpy)(CO)3CN] electrocatalyst, our CN-bridged binuclear complex is a more efficient electrocatalyst for CO2 reduction using H2O as a proton source. In addition, we report a photochemical CO2 reduction to CO using the dimanganese complex under 395 nm irradiation.


 Please wait while we load your content...
Please wait while we load your content...
