Non-innocent PNN ligand is important for CO oxidation by N2O catalyzed by a (PNN)Ru–H pincer complex: insights from DFT calculations†
Abstract
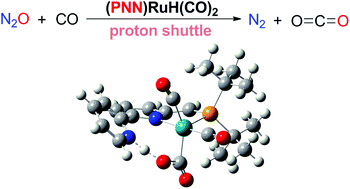
Milstein et al. developed an efficient and mild method for CO oxidation by N2O to give CO2 and N2 catalyzed by a (PNN)Ru–H pincer complex. To gain mechanistic information on this catalytic transformation, the reaction mechanism has been studied using density functional theory (DFT) calculations. It was found that the catalytic cycle for CO oxidation by N2O proceeds in three stages: N2O activation to form a (PNN)Ru–OH intermediate, CO insertion into the Ru–OH bond to form a (PNN)Ru–COOH intermediate and CO2 release from (PNN)Ru–COOH. In the CO2 release stage, CO2 is not released via a β-H elimination mechanism as proposed in experiments, instead it is released via a deprotonation mechanism. The calculations demonstrated that the Ru–H bond of the catalyst plays an important role in facilitating the activation of N2O, which is the rate-determining step for the whole catalytic cycle, and the non-innocent PNN ligand is very important for CO oxidation by N2O. Our theoretical results are consistent with the experimental observations and could help design highly efficient catalysts for N2O activation.


 Please wait while we load your content...
Please wait while we load your content...