All inorganic coordination polymers have been made possible with the m-carboranylphosphinate ligand†
Abstract
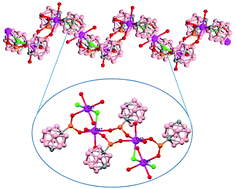
New examples of 1D coordination polymers (CPs) and complexes containing the purely inorganic carboranylphosphinate ligand [1-OPH(O)-1,7-closo-C2B10H11]− are reported. The reaction of Na[1-OPH(O)-1,7-closo-C2B10H11] salt with MCl2 (M = Mn, Co, Ni, Cu, Zn and Cd) in MeOH or EtOH leads to compounds 1–8. All compounds have been exhaustively characterized by analytical and spectroscopic techniques. X-ray analysis and spectroscopy characterization revealed the differences between the isolated compounds: 1D polymeric chains (CPs) with carboranylphosphinate ligand bridges have been obtained with MnII, CdII or ZnII centres, whereas compounds with low nuclearity have been isolated with CuII, CoII and NiII. No polymeric structures were obtained in the CoII and NiII complexes due to the higher affinity of these metals for water than that for the m-carboranylphosphinate and accordingly, these complexes generate supramolecular hydrophobic/hydrophilic structures. The reactivity of manganese polymer 1 with water leads to the breakage of the polymer with the formation of a new mononuclear compound 2, and that in methanol leads back to the initial polymer 1. However, the reactivity of polymer 1 with 2,2′-bpy maintains the core present in the initial polymer, leading to the CP 3, which in methanol/water medium produces species of lower nuclearity. The magnetic properties of the compounds studied show weak antiferromagnetic coupling.


 Please wait while we load your content...
Please wait while we load your content...