Phenoxide chelated Ir(iii) N-heterocyclic carbene complexes: synthesis, characterization, and evaluation of their in vitro anticancer activity†
Abstract
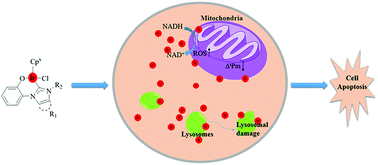
Twelve novel half-sandwich IrIII–NHC complexes [(η5-Cpx)Ir(C^O)Cl] were synthesized and characterized. These complexes showed higher cytotoxic activity toward A549 cells and HeLa cells than cisplatin. An increase in the number of contained phenyl groups was related to better anticancer activity. The reaction of complexes with nucleobases 9-MeA, nucleobases 9-EtG, plasmid DNA and CT-DNA showed no significant effects. These complexes captured hydrogen from NADH and converted it to NAD+, which produced the reactive oxygen species (ROS). ROS led to a decrease in the mitochondrial membrane potential and lysosomal damage, finally inducing apoptosis.


 Please wait while we load your content...
Please wait while we load your content...