Palladium alkyl complexes with a formazanate ligand: synthesis, structure and reactivity†
Abstract
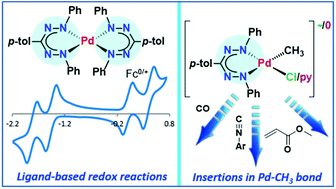
Palladium(II) complexes with a bidentate, anionic formazanate ligand are described. Attempts to prepare mono(formazanate) palladium alkyl complexes often leads to the homoleptic bis(formazanate) complex, which shows rich electrochemistry due to the redox-active nature of the ligands. Performing salt metathesis between the precursor [Pd(COD)(CH3)Cl] and the potassium salt of the ligand in the presence of tetrabutylammonium chloride yields a square planar mono(formazanate) palladate complex through coordination of chloride anion. Ligand exchange allows binding of unsaturated molecules and evaluation of the reactivity of the Pd–CH3 fragment. Using this approach, insertion reactions of CO, isocyanide and methyl acrylate into the Pd–CH3 bond are demonstrated.


 Please wait while we load your content...
Please wait while we load your content...