Impact of copper and iron binding properties on the anticancer activity of 8-hydroxyquinoline derived Mannich bases†
Abstract
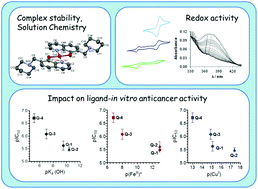
The anticancer activity of 8-hydroxyquinolines relies on complex formation with redox active copper and iron ions. Here we employ UV-visible spectrophotometry and EPR spectroscopy to compare proton dissociation and complex formation processes of the reference compound 8-hydroxyquinoline (Q-1) and three related Mannich bases to reveal possible correlations with biological activity. The studied derivatives harbor a CH2–N moiety at position 7 linked to morpholine (Q-2), piperidine (Q-3), and chlorine and fluorobenzylamino (Q-4) substituents. Solid phase structures of Q-3, Q-4·HCl·H2O, [(Cu(HQ-2)2)2]·(CH3OH)2·Cl4·(H2O)2, [Cu(Q-3)2]·Cl2 and [Cu(HQ-4)2(CH3OH)]·ZnCl4·CH3OH were characterized by single-crystal X-ray diffraction analysis. In addition, the redox properties of the copper and iron complexes were studied by cyclic voltammetry, and the direct reaction with physiologically relevant reductants (glutathione and ascorbic acid) was monitored. In vitro cytotoxicity studies conducted with the human uterine sarcoma MES-SA/Dx5 cell line reveal the significant cytotoxicity of Q-2, Q-3, and Q-4 in the sub- to low micromolar range (IC50 values 0.2–3.3 μM). Correlation analysis of the anticancer activity and the metal binding properties of the compound series indicates that, at physiological pH, weaker copper(II) and iron(III) binding results in elevated toxicity (e.g.Q4: pCu = 13.0, pFe = 6.8, IC50 = 0.2 μM vs.Q1: pCu = 15.1, pFe = 13.0 IC50 = 2.5 μM). Although the studied 8-hydroxyquinolines preferentially bind copper(II) over iron(III), the cyclic voltammetry data revealed that the more cytotoxic ligands preferentially stabilize the lower oxidation state of the metal ions. A linear relationship between the pKa (OH) and IC50 values of the studied 8-hydroxyquinolines was found. In summary, we identify Q-4 as a potent and selective anticancer candidate with significant toxicity in drug resistant cells.


 Please wait while we load your content...
Please wait while we load your content...