A dual response fluorescent sensor for HNO and S2− ions using a Cu(ii) complex based probe assisted by detailed DFT studies†
Abstract
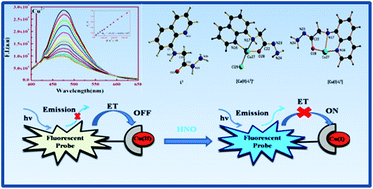
A Cu(II) based sensor (1) prepared by the complexation between (quinolin-8-ylamino)-acetic acid hydrazide (L2) and Cu2+ ions has been developed for a highly sensitive and selective recognition of HNO and S2− over other biologically abundant anions with prominent enhancement in absorption and emission intensities. The sensor (1) shows weak fluorescence due to ET (electron transfer) but upon addition of HNO and S2− a large enhancement in the fluorescence intensity (F.I.) was observed over other possible competitive anions on the basis of reduction of Cu(II) to Cu(I) and formation of CuS, respectively. The 1 : 1 complexation was characterized by mass spectrometry (MS), elemental analysis and Job's plot. The corresponding Kf value was evaluated to be (4.934 ± 0.05) × 104 M−1 for Cu2+ from UV-Vis absorption titration. Quantum yields of L2 and [Cu-L2 + S2−] and [Cu-L2 + HNO] complexes in acetonitrile (CH3CN) are found to be 0.107, 0.09 and 0.07, respectively, using quinine sulphate as the standard.


 Please wait while we load your content...
Please wait while we load your content...