Computational mechanistic elucidation of the rare earth metal-mediated cycloamidination of aminoalkenes with nitriles†
Abstract
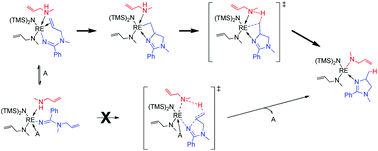
A detailed computational probe of the rare earth metal-mediated intramolecular amidination of aminoalkenes with nitriles by an archetypical [Y{N(SiMe3)2}3] precatalyst is presented. The mechanistic picture derived from smooth energy profiles, acquired by employing a reliable computational protocol applied to a realistic catalyst model, conforms to available experimental data that includes a significant primary KIE. Sequential Y–N silylamide aminolysis transforms the precatalyst into a multitude of silylamide/amide compounds, of which the bis-amine coordinated [Y{N(SiMe3)2}(NRR′)2] is the most abundant, capable of promoting cycloamidination. Nitrile insertion is irreversible and readily furnishes the κ2-N-amidinate yttrium intermediate, which readily rearranges into more stable isomers featuring κ2-N,Δ and κ1-N(imine) amidinate ligations. Its bis-amine adduct likely represents the catalyst resting state. The alkenylamidine becomes accessible through kinetically affordable Y–N amidinate bond protonolysis, which can best be viewed as a kinetically mobile equilibrium that favours the amidinate. The generation of 2-imidazoline product via N–C bond forming amidinate cyclisation favours a stepwise σ-insertive cyclisation/Y–C alkyl aminolysis sequence over an otherwise kinetically prohibitive proton-triggered concerted N–C/C–H bond forming process. The operative σ-insertive pathway entails reversible olefin 1,2-insertion followed by turnover-limiting Y–C alkyl aminolysis at the short-lived 4-imidazolylalkyl intermediate. The DFT estimated primary KIE associated with aminolysis is gratifyingly close to the observed value, thereby supporting the derived mechanistic view.


 Please wait while we load your content...
Please wait while we load your content...