Outerly functionalized and non-functionalized boron clusters intercalated into layered hydroxides with different modes of binding: materials for superacid storage
Abstract
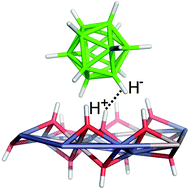
Two binary boron hydrides (NH4)2B10H10 and Na2B12H12 and mono- and dicarboxy p- and m-carboranes (namely, 1-(COOH)-closo-1,7-C2B10H11, 1,12-(COOH)2-closo-1,12-C2B10H10 and 1,7-(COOH)2-closo-1,7-C2B10H10) were intercalated into ZnAl-layered double hydroxides (ZnAl-LDH) and into Zn5(OH)8(NO3)2·2H2O. The formed compounds were characterized using elemental analysis, thermogravimetry analysis, X-ray powder diffraction, infrared spectroscopy and solid state NMR. All the intercalated boron compounds are present in the interlayer space of the layered hosts as anions. It is presumed that in the case of B10H102−, B12H122− and 1,12-(COO)2-closo-1,12-C2B10H102−, the guest molecules form a monolayer, whereas in the case of 1-(COO)-closo-1,7-C2B10H111− and 1,7-(COO)2-closo-1,7-C2B10H102− a bilayer arrangement is more probable. In the case of 1,7-(COO)2-closo-1,7-C2B10H102−, the guest molecules are strongly interdigitated resulting in lowering of the interlayer distance. Two different modes of binding were found. Whereas the carboxylate derivatives of p- and m-carboranes are bonded through classical hydrogen bonds, the corresponding parent borane anions interact with the host structures by mainly dihydrogen bonding. In effect, both kinds of hydrogen bonding are mainly of an electrostatic nature. The dihydrogen bond is detected, e.g. in crystal engineering, and represents a driving force for interactions of boranes with biomolecules. Since the latter dicarboxylic acids were found to be superacids, their interactions with the host structures should be stronger than in the case of the benzoic and terephthalic acid intercalates.


 Please wait while we load your content...
Please wait while we load your content...