Mixed-valence dimolybdenum complexes containing hard oxo and soft carbonyl ligands: synthesis, structure, and electrochemistry of Mo2(O)(CO)2(μ-κ2-S(CH2)nS)2(κ2-diphosphine)†
Abstract
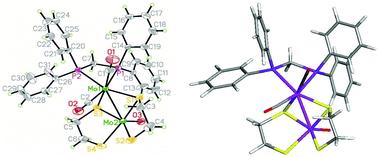
Mixed-valence dimolybdenum complexes Mo2(O)(CO)2{μ-κ2-S(CH2)nS}2(κ2-Ph2P(CH2)mPPh2) (n = 2, 3; m = 1, 2) (1–4) have been synthesized from one-pot reactions of fac-Mo(CO)3(NCMe)3 and dithiols, HS(CH2)nSH, in the presence of diphosphines. The dimolybdenum framework is supported by two thiolate bridges, with one molybdenum carrying a terminal oxo ligand and the second two carbonyls. The dppm (m = 1) products exist as a pair of diastereomers differing in the relative orientation of the two carbonyls (cis and trans) at the Mo(CO)2(dppm) center, while dppe (m = 2) complexes are found solely as the trans isomers. Small amounts of Mo(CO){κ3-S(CH2CH2S)2}(κ2-dppe) (5) also result from the reaction using HS(CH2)2SH and dppe. The bonding in isomers of 1–4 has been computationally explored by DFT calculations, trans diastereomers being computed to be more stable than the corresponding pair of cis diastereomers for all. The calculations confirm the existence of Mo![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O and Mo–Mo bond orders and suggest that the new dimeric compounds are best viewed as Mo(V)–Mo(I) mixed-valence systems. The electrochemical properties of 1 have been investigated by CV and show a reversible one-electron reduction associated with the Mo(V) centre, while two closely spaced irreversible oxidation waves are tentatively assigned to oxidation of the Mo(I) centre of the two isomers as supported by DFT calculations.
O and Mo–Mo bond orders and suggest that the new dimeric compounds are best viewed as Mo(V)–Mo(I) mixed-valence systems. The electrochemical properties of 1 have been investigated by CV and show a reversible one-electron reduction associated with the Mo(V) centre, while two closely spaced irreversible oxidation waves are tentatively assigned to oxidation of the Mo(I) centre of the two isomers as supported by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...