Exploring diversity in platinum(iv) N-heterocyclic carbene complexes: synthesis, characterization, reactivity and biological evaluation†
Abstract
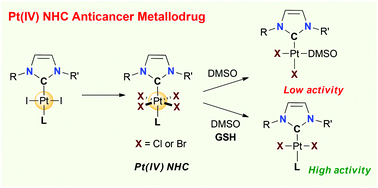
Platinum(IV) complexes stabilized by N-heterocyclic carbene ligands of the type [(NHC)PtX4L], where L is a neutral nitrogen-based ligand and X is a halide anion (Br, Cl), were prepared by using straightforward and high-yielding synthetic routes and the scope was extended to amphiphilic derivatives. The complexes were fully characterized and the molecular structure of the three derivatives was determined by single-crystal X-ray analyses. The complexes demonstrated in vitro antiproliferative activities against several cancer cell lines. In particular, a representative Pt(IV) complex, namely, [(NHC)PtCl4(pyridine)], displayed efficient antiproliferative activity against cisplatin-resistant cancer cells. These results were correlated with their physicochemical properties, namely, solubility, stability and redox behavior by means of UV-vis spectroscopy, NMR or cyclic voltammetry, whereas in DMSO/water, these Pt(IV) complexes transform into biologically less active cis[(NHC)PtX2(DMSO)] species, in the presence of a bioreductant such as glutathione which quickly leads to the formation of a biologically active trans[(NHC)PtX2L] complex. Overall, these data show that NHC-Pt(IV) compounds are good candidates as anti-cancer prodrugs.


 Please wait while we load your content...
Please wait while we load your content...