The new diphosphanylphosphido complexes of tungsten(vi) and molybdenum(vi). Their synthesis, structures and properties†
Abstract
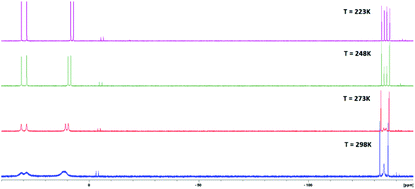
We report on the reactivity of R2P–P(Li)–PR′2 (R = tBu, iPr, R′ = NEt2, iPr) towards diimido complexes [(dippN)2MCl2·dme] (M = Mo, W and dipp = 2,6-iPr2C6H3). A series of new complexes with diphosphanylphosphido ligands R2P–P–PR′2 were isolated. The solid-state structures of [(dippN)2M(Cl)(1,2-η-iPr2P–P–PiPr2)] (2Mo and 2W) and [(dippN)2M(Cl){1,2-η-tBu2P–P–P(NEt2)2}] (3Mo and 3W) were established by single-crystal X-ray diffraction analysis and indicate a side-on geometry of the R2P–P–PR′2 moiety. 3W and 3Mo are the first triphosphorus complexes with the amido ligand NEt2 on the P atom. [(dippN)2M(Cl)(1,2-η-tBu2P–P–PtBu2)] (1Mo and 1W) and 3Mo and 3W display similar side-on geometry in solution and in the solid state. By contrast, 2Mo and 2W reveal a dynamic behavior in solution. For the first time, the reactivity of diphosphanylphosphido complexes towards different nucleophiles was studied. The complexes react with the phosphorus nucleophile Ph2PLi, yielding phosphanylphosphinidene complexes [(dippN)2M(Cl)(η2-P-PR2)]− Li+ (M = Mo, W) together with related diphosphanes R′2P–PPh2. Carbon nucleophile MeLi does not yield [(dippN)2M(Cl)(η2-P-PR2)]− Li+ but substitutes a Cl ligand at the metal center. Moreover, we compare the coordination of the R2P–P–PR′2 moiety to different metal centers based on DFT methods.


 Please wait while we load your content...
Please wait while we load your content...