Heterometallic CuII/LnIII polymers active in the catalytic aerobic oxidation of cycloalkenes under solvent-free conditions†
Abstract
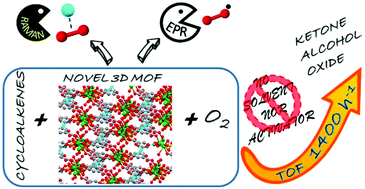
Heterometallic 3d–4f inorganic polymers were prepared using 3,5 pyridinedicarboxylic acid (H2PDC), {[CuLn2(PDC)2(SO4)2(H2O)6]·H2O}n (Ln: SmIII, CuSmPDC, EuIII, CuEuPDC, GdIII, and CuGdPDC). These catalysts are active in the aerobic oxidation of cycloalkenes under solvent-free conditions, with a conversion for the oxidation of cyclohexene of 71% after one hour of the reaction, and a TOF value of 1438 h−1 for CuSmPDC. On the other hand, the oxidation of cycloheptene and cyclooctene exhibited slightly lower conversions of 52% and 47%, and TOF values of 1053 and 159 h−1 after 1 and 6 hours of the reaction, respectively. The radical mechanism for the oxidation reaction of cyclohexene was assessed by Raman and EPR spectroscopy. The first evidenced the formation of Cu–O2 adducts and the second permitted is to observe the presence of the oxygen centered radical species, which act as initiators of the reaction chain to generate the products. An increase in the temperature of the reaction correlates with the adduct formation, and with the enhancement of the oxidation reaction.


 Please wait while we load your content...
Please wait while we load your content...