Carbonyl and ester C–O bond hydrosilylation using κ4-diimine nickel catalysts†
Abstract
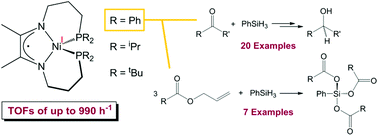
The synthesis of alkylphosphine-substituted α-diimine (DI) ligands and their subsequent addition to Ni(COD)2 allowed for the preparation of (iPr2PPrDI)Ni and (tBu2PPrDI)Ni. The solid state structures of both compounds were found to feature a distorted tetrahedral geometry that is largely consistent with the reported structure of the diphenylphosphine-substituted variant, (Ph2PPrDI)Ni. To explore and optimize the synthetic utility of this catalyst class, all three compounds were screened for benzaldehyde hydrosilylation activity at 1.0 mol% loading over 3 h at 25 °C. Notably, (Ph2PPrDI)Ni was found to be the most efficient catalyst while phenyl silane was the most effective reductant. A broad scope of aldehydes and ketones were then hydrosilylated, and the silyl ether products were hydrolyzed to afford alcohols in good yield. When attempts were made to explore ester reduction, inefficient dihydrosilylation was noted for ethyl acetate and no reaction was observed for several additional substrates. However, when an equimolar solution of allyl acetate and phenyl silane was added to 1.0 mol% (Ph2PPrDI)Ni, complete ester C–O bond hydrosilylation was observed within 30 min at 25 °C to generate propylene and PhSi(OAc)3. The scope of this reaction was expanded to include six additional allyl esters, and under neat conditions, turnover frequencies of up to 990 h−1 were achieved. This activity is believed to be the highest reported for transition metal-catalyzed ester C–O bond hydrosilylation. Proposed mechanisms for (Ph2PPrDI)Ni-mediated carbonyl and allyl ester C–O bond hydrosilylation are also discussed.


 Please wait while we load your content...
Please wait while we load your content...