Titanium-oxo clusters functionalized with catecholate-type ligands: modulating the optical properties through charge-transfer transitions†
Abstract
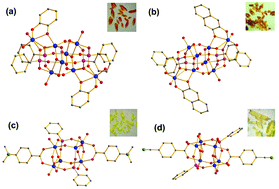
Four phenylphosphonate-stabilized titanium-oxo clusters with varying functional ligands, namely, [Ti8(μ3-O)2(μ2-O)2(μ2-OiPr)4(OiPr)8(O3PC6H5)4(cat)2] (cat = catecholate), [Ti8(μ3-O)2(μ2-O)2(μ2-OiPr)4(OiPr)8(O3PC6H5)4(O2C10H6)2] (O2C10H6 = naphthalene-2,3-diolate), [Ti6(μ3-O)2(μ2-O)2(μ2-OiPr)4(OiPr)6(O3PC6H5)2(4-DMAB)2] (4-DMAB = 4-dimethylaminobenzoate), and [Ti6(μ3-O)2(μ2-O)2(μ2-OiPr)4(OiPr)6(O3PC6H5)2(4-CBA)2] (4-CBA = 4-cyanobenzoate) were synthesized and structurally characterized. The introduction of catecholate ligands effectively extended the visible absorption region up to 670 nm and reduced the band gap to 2.1 eV. DFT calculations revealed that the ligand-based energy levels could effectively modify the band structure of titanium-oxo clusters. The ligand-to-core charge transfer (LCCT) transition from the functional ligands to the cluster core is responsible for the low-energy charge transfer states. Photoelectrochemical and photocatalytic experiments show that functional ligands have significant influence on the physicochemical properties of titanium-oxo clusters.


 Please wait while we load your content...
Please wait while we load your content...