Complexes between neutral oxyacid beryllium salts and dihydrogen: a possible way for hydrogen storage?†
Abstract
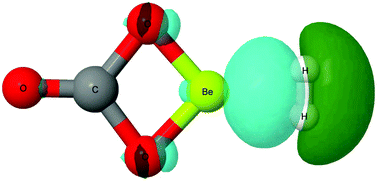
Accurate ab initio calculations reveal that oxyacid beryllium salts yield rather stable complexes with dihydrogen. The binding energies range between −40 and −60 kJ mol−1 for 1 : 1 complexes, remarkably larger than others previously reported for neutral H2 complexes. The second H2 molecule in 1 : 2 complexes is again strongly bound (between −18 and −20 kJ mol−1). The incoming H2 molecules in 1 : n complexes (n = 3–6) are more weakly bound, confirming the preference of Be for tetracoordinated arrangements.
- This article is part of the themed collection: Organometallic and coordination chemistry of the s-block metals


 Please wait while we load your content...
Please wait while we load your content...