Varied spin crossover behaviour in a family of dinuclear Fe(ii) triple helicate complexes†
Abstract
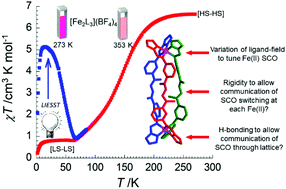
Dinuclear triple-helicate complexes of the formula [Fe2L3](BF4)4·solv (solv = CH3CN, CHCl3, H2O) have been synthesised and structurally characterised. The bis-bidentate ligands, L, present either strong-field 2-pyridylimine (1) or weaker-field 2-imidazolylimine (2) and 4-imidazolylimine (3) coordination spheres about Fe(II) centres in an octahedral geometry. Whereas 1 is pervasively diamagnetic, spin crossover (SCO) behaviour is observed in 2 and 3 and has been studied using variable-temperature structural, UV-visible spectroscopic, magnetic and photo-magnetic techniques. Variable-temperature (1.8–400 K) magnetic-susceptibility measurements reveal the T1/2 values of 2 and 3 to be strongly dependent upon the solvent and degree of solvation. Photomagnetic studies at 10 K under white-light irradiation revealed an inefficient photo-induced SCO in 2, but full switching in 3.


 Please wait while we load your content...
Please wait while we load your content...