Playing with Pearson's concept: orthogonally functionalized 1,4-diaza-1,3-butadienes leading to heterobinuclear complexes†
Abstract
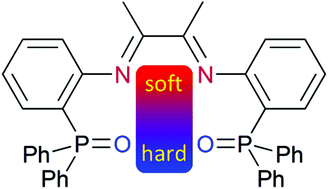
By reacting 1,2-diketones and ortho- diphenylphosphinoyl aniline in the presence of zinc(II) as a templating agent, cationic zinc(II) complexes of novel phosphine oxide functionalized 1,4-diaza-1,3-butadiene ligands are acessible. Herein the zinc(II) site is bound to all four donor atoms of the ligand. Depending on the flexibility of the 1,4-diaza-1,3-butadiene backbone, the bonds to zinc(II) from the 1,4-diaza-1,3-butadiene donors can be broken. Reaction with oxalate cleaves the zinc(II) coordination completely and makes accessible the free ligands possessing orthogonal (N,N: soft; O,O: hard) sets of donor sites. This allows for the specific coordination of soft and hard Lewis acids and thus for the generation of heterobimetallic complexes, here exemplarily shown for the combination of palladium(II) (soft) and zinc(II) (hard) centres.


 Please wait while we load your content...
Please wait while we load your content...