An octahedral tetrachlorido Fe(ii) complex with aminopyrazinium ligands from a serendipitous redox synthesis exhibiting magnetic exchange through non-covalent 3-D architectures†
Abstract
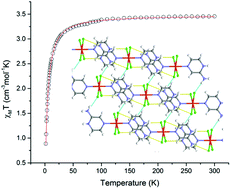
An air stable, neutral Fe(II) complex with four equatorial chlorido ligands has been stabilised through a serendipitous redox process and in situ ligand protonation. A three-dimensional non-covalent network composed of halogen bonding and π–π stacking promotes magnetic exchange interactions though the lattice. The electronic structure has been investigated using DFT.


 Please wait while we load your content...
Please wait while we load your content...