Complexation-assisted reduction: complexes of glutaroimide-dioxime with tetravalent actinides (Np(iv) and Th(iv)) †
Abstract
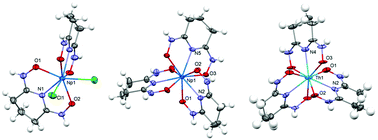
Glutaroimide-dioxime forms strong complexes with tetravalent Th(IV) and Np(IV) in aqueous solution. In conjunction with literature data on the complexation of glutaroimide-dioxime with other metal ions, it was found that the complexes become weaker as the effective charge density on the metal ions decreases: V5+ > Th4+ ≈ Fe3+ > UO22+ > Eu3+/Nd3+ > Cu2+ > Pb2+ > NpO2+ > Ca2+/Mg2+. In the glutaroimide-dioxime complexes with Th(IV) and Np(IV), deprotonation of the imide group and relocation of the two hydrogen atoms from oxygen to nitrogen of the oxime groups result in a large conjugated system (–O–N–C–N–C–N–O–) that coordinates strongly to the metal center in a tridentate mode via the central imide nitrogen atom and the two oxime oxygen atoms. Because the stability of glutaroimide-dioxime complexes with Np(IV) is much higher than those with Np(V), the redox potential of the Np(V)/Np(IV) couple is expected to be shifted significantly. As a result, crystals of glutaroimide-dioxime complexes with Np(IV) were readily obtained from initial solutions containing Np(V). A mechanism of complexation-assisted reduction integrating the thermodynamic and structural data from this work is discussed.


 Please wait while we load your content...
Please wait while we load your content...