Novel paramagnetic clays obtained through intercalation of Gd3+-complexes†
Abstract
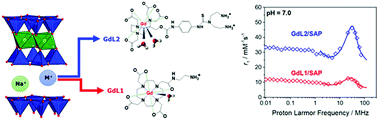
Novel paramagnetic lanthanide-exchanged saponite clays, bearing in the interlamellar region positively charged Gd3+-complexes, were synthesized using a hydrothermal approach followed by a classical ion exchange reaction. A detailed characterization was performed to assess the physico-chemical properties of the samples, which showed a hydrodynamic diameter between 50 and 90 nm and good thermal stability. 1H-NMR relaxometric studies in aqueous solution as a function of magnetic field and temperature were carried out to evaluate the local chemical environment of the intercalated paramagnetic centres and their interaction with water molecules. The data indicate a strong interaction of the confined complexes with the lamellae, resulting in a restriction of the local rotational dynamics and of the exchange process of water molecules between the inner coordination sphere of Gd3+ and the bulk. The stability over time in aqueous solutions of varying complexity was also studied by relaxometric techniques.


 Please wait while we load your content...
Please wait while we load your content...