Synthesis and crystal structure of solvent-free dodecahydro closo-dodecaborate of nickel, NiB12H12†
Abstract
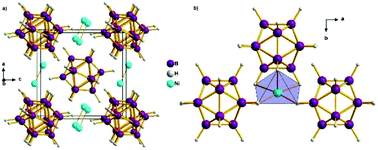
Nickel closo-dodecaborate NiB12H12 was prepared by mechanosynthesis (ball milling) of mixtures of Na2B12H12 + NiCl2 followed by hydration and drying under dynamic vacuum. The crystal structures of hydrated and anhydrous closo-dodecaborates were characterized by temperature dependent synchrotron radiation X-ray powder diffraction, ab initio calculations, thermal analysis and infrared spectroscopy. Three different water containing complexes were found: a homoleptic octahedral complex in Ni(H2O)6B12H12 crystallizing in two different deformation variants of a complex centred closo-dodecaborate cube, and a heteroleptic octahedral complex in Ni(H2O)4B12H12 containing four water molecules and two hydrogens and centring also a deformed closo-dodecaborate cube. Anhydrous nickel closo-dodecaborate was obtained by drying the hydrated sample under dynamic vacuum. It crystallizes with bcc packing of B12H122− anions and Ni2+ is disordered close to the triangular face of the tetrahedral interstice coordinated by a H5 square pyramid.


 Please wait while we load your content...
Please wait while we load your content...