Kinetics and mechanism of the chromium(vi) catalyzed decomposition of hypochlorous acid at elevated temperature and high ionic strength
Abstract
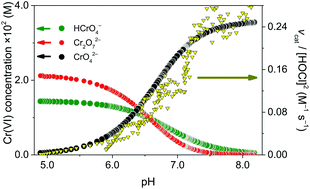
An important reaction step in the industrial production of NaClO3 (electrochemical chlorate process) is the thermal decomposition of HOCl/OCl− to yield ClO3− and Cl−. It is widely accepted that this reaction is accelerated by aqueous chromium(VI) species. A detailed kinetic study was conducted under industrially relevant conditions, i.e. at high ionic strength (6.0 M) and elevated temperature (80 °C), to investigate this phenomenon. The decomposition of hypochlorous acid was followed in the presence of Cr(VI) or phosphate (PO43−) or without any additive. In addition to the beneficial pH buffering effect of Cr(VI), the CrO42− form of chromium(VI) was found to slightly catalyze the decomposition of hypochlorous acid. The overall rate of HOCl decomposition can be expressed as −dcHOCl/dt = kdec[HOCl]2[OCl−] + kcat[HOCl]2[CrO42−]. The corresponding rate constants were determined, kdec = 9.4 ± 0.1 M−2 s−1 and kcat = 4.6 ± 0.8 M−2 s−1, and mechanistic interpretation of the catalytic rate law is given. The contribution of the catalytic path to the overall rate of decomposition changes from ca. 30% at pH = 8 to ca. 70% at pH = 6.


 Please wait while we load your content...
Please wait while we load your content...