Hybrid mesoporous organosilicas with molecularly imprinted cavities: towards extended exposure of active amino groups in the framework wall†
Abstract
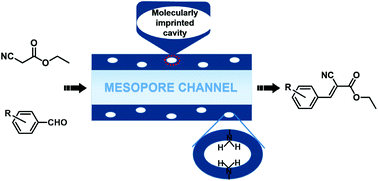
Hybrid molecularly imprinted mesoporous silicas were synthesized by co-condensation of tetraethoxysilane and functional organosilica precursors of HQP and BPAP, in which hydroquinone (HQ) and bisphenol A (BPA) were linked as imprinting molecules. Owing to the existence of a thermally reversible covalent bond of carbamate (–NH–COO–), the imprinting molecules could be eliminated under thermal treatment and molecularly imprinted cavities were formed in the framework wall. All of these materials were used to catalyze heterogeneous Knoevenagel reactions and proved to exhibit higher catalytic conversion and turnover frequency (TOF) number compared with the materials with imprinting molecules, which is attributed to the presence of amino groups with higher basicity and molecularly imprinted cavities. Importantly, compared with amino functionalized SBA-15 materials, the decisive role of molecularly imprinted cavities in the enhanced accessibility of amino groups in a mesoporous framework was further confirmed. Moreover, the size of imprinting molecules has an influence on the catalytic conversion and TOF values: imprinting molecules with relatively bulkier size tend to provide higher accessibility to the active sites. The amino groups in the framework are extremely stable during the reaction procedure and recycling process.


 Please wait while we load your content...
Please wait while we load your content...