Novel tetra-propyl/butylammonium encapsulated Keggin-type polyoxotungstates: synthesis, structural characterization, and catalytic capability in oxidative cleavage of unsaturated fatty acids†
Abstract
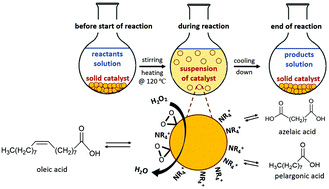
Four tungsten oxide (TO)-based catalysts, (1) TO (without any organic moiety), (2) TO-TMA, (3) TO-TPA, and (4) TO-TBA (TMA: tetramethylammonium, TPA: tetrapropylammonium, and TBA: tetrabutylammonium cations) were straightforwardly prepared via a mild hydrothermal approach, based on oxidative dissolution of tungsten bare powder, followed by a facile encapsulation of the tetraalkylammonium cations (NR4+). The significant impact of the NR4+ cations on crystallization of the amorphous and metastable peroxotungstic species, formed during the synthesis, has been discussed through different characterization techniques including XRD, FTIR, TGA and DTG, TEM, EDS, N2 adsorption/desorption isotherms, zeta potential measurement, and elemental analysis. TO-TPA and TO-TBA were composed of two novel hybrid inorganic–organic polyoxotungstates, [(CH3CH2CH2)4N]2.75[H5.25W12O40]·7.42H2O and [(CH3CH2CH2CH2)4N]3.31[H4.69W12O40]·1.08H2O, respectively. The capability of the synthesized catalysts in oxidation of unsaturated fatty acids (UFAs) has been examined in the reaction of oleic acid, the most abundant UFA, with H2O2. This is the first report on employing heterogeneous Keggin clusters of tungsten oxide in the oxidative cleavage of UFAs. The catalysts exhibited excellent efficiencies, particularly TO-TPA and TO-TBA, owing to the strong and organo-protected acidity of their Keggin structures, which resulted in full conversion of oleic acid and superior yields of the desired products. Despite their pseudo-homogeneous behavior in the reaction, the NR4+-encapsulated catalysts demonstrated efficient recovery and steady reusability without any significant decay in their activities up to at least 4 cycles.


 Please wait while we load your content...
Please wait while we load your content...