A(VO2F)(SeO3) (A = Sr, Ba) and Ba(MOF2)(TeO4) (M = Mo, W): first examples of alkali-earth selenites/tellurites with a fluorinated d0-TM octahedron†
Abstract
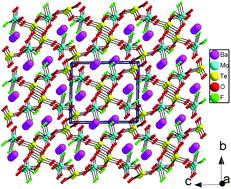
Four new compounds of A(VO2F)(SeO3) (A = Sr 1, Ba 2) and Ba(MOF2)(TeO4) (M = Mo 3, W 4) oxyfluorides have been synthesized successfully by hydrothermal reactions. They represent the first examples of alkali-earth selenites or tellurites with a fluorinated d0-TM octahedron. Their crystal structures were determined by single-crystal X-ray diffraction. They exhibit two different types of structures. Compounds 1 and 2 are isostructural and crystallize in the orthorhombic crystal system with the space group of Pbca (no. 61). Their structures feature a 3D network composed of 0D [V2O4F2(SeO3)2]4− dimers which are further bridged by Sr2+ or Ba2+ cations. Isostructural compounds 3 and 4 are crystallized in the monoclinic crystal system with the space group of P21/c (no. 14). Their structures can be described as 2D [(MOF2)(TeO4)]2− (M = Mo, W) anionic layers separated by Ba2+ cations. Out-of-center distortion studies show that the magnitude of the distortion of the (MOxF6−x) octahedron is comparable to that of MO6 (M = d0 transition metal). Infrared spectroscopy, thermal stability, optical property studies and theoretical calculations based on density functional theory methods were also performed.


 Please wait while we load your content...
Please wait while we load your content...