A Cu(ii) metal–organic framework with significant H2 and CO2 storage capacity and heterogeneous catalysis for the aerobic oxidative amination of C(sp3)–H bonds and Biginelli reactions†
Abstract
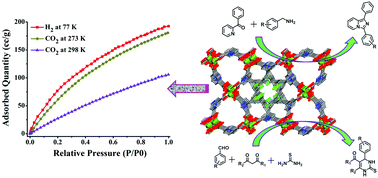
A Cu(II) metal–organic framework, {[Cu2(L)(H2O)2]·(5DMF)(4H2O)}n (1), has been synthesized using an angular tetracarboxylic acid ligand (H4L) incorporating both trifluoromethyl (–CF3) and amine (–NH2) groups. Notably, the framework possesses high water and thermal stability. At atmospheric pressure, the activated framework 1′ exhibits substantially high amounts of CO2 (35.5 and 20.8 wt% at 273 and 298 K respectively) and H2 (1.72 wt% at 77 K) adsorption. Also, 1′ exhibits excellent catalytic activity for the condensation–cyclization reaction between 2-benzoyl pyridine and different benzylamines via oxidative amination of the C(sp3)–H bond to form 1,3-diarylated imidazo[1,5-a]pyridines under mild aerobic conditions. In addition to this, 1′ shows excellent heterogeneous catalytic activity in Biginelli reactions. The solid catalyst could be recycled several times without significant loss in the catalytic activities.


 Please wait while we load your content...
Please wait while we load your content...