Self-assembly of heteroleptic dinuclear silver(i) complexes bridged by bis(diphenylphosphino)ethyne†
Abstract
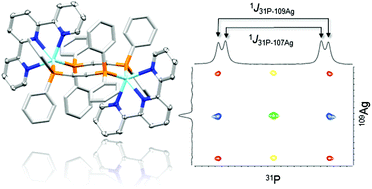
We present a study of the self-assembling behaviour in solution of Ag[PF6] with one equivalent of an N^N ligand (N^N = 6,6′-dimethyl-2,2′-bipyridine (6,6′-Me2bpy) or 2,2′:6′,2′′-terpyridine (tpy)), together with either 0.5 or one equivalent of the bridging ligand bis(diphenylphosphino)ethyne (dppa). Each product contains a dinuclear cation, with one or two dppa ligands bridging the two Ag+ centres; 6,6′-Me2bpy and tpy, respectively, act as chelating ligands. The compounds have been analysed in solution using NMR spectroscopic methods (1H, 1H{31P}, COSY, NOESY, 13C, HMQC and HMBC), including room and low temperature measurements. 31P{1H} NMR spectra recorded at different temperatures allow a deeper insight into the dynamic equilibrium processes. In addition, solution 31P–109Ag HSQC spectroscopic measurements were performed and show, inter alia, the ratio of the splitting in the F1 dimension and the 1J31P–109Ag coupling constant in the fully coupled HSQC. Single crystal X-ray diffraction yielded two pseudo-polymorphic structures for the doubly-bridged [Ag2(dppa)2(tpy)2][PF6]2 and one for the singly-bridged [Ag2(dppa)(6,6′-Me2bpy)2][PF6]2.


 Please wait while we load your content...
Please wait while we load your content...