Palladium pincer-type complexes and zwitterionic sulfur adducts of pyridine-bridged bis(1,2,3-triazolin-5-ylidenes): syntheses, characterizations and catalytic applications†
Abstract
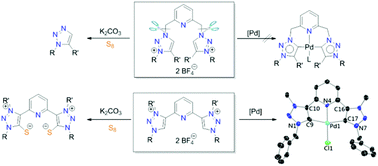
Four pyridine-bridged bis(1,2,3-triazoles) A/B and C/D have been prepared by “click” reactions. Methylations/ethylations of A/B and C/D employing Meerwein's salts led to the formation of the corresponding dicationic salts 1/2 and 3/4 with pyridine moieties surviving from the alkylations. Interestingly, the reactions of “N-linked” salts 1/2 with K2CO3 in the presence of elemental sulfur did not yield the mesoionic carbene–sulfur betaine adducts but unexpectedly afforded 1,5-disubstituted triazoles 5/6, which may produce pyridine thioaldehydes as by-products. In contrast, in the case of “C-linked” salts 3/4, the reactions to synthesize carbene–sulfur zwitterions 9/10 proceeded smoothly. Employing a silver–carbene transfer protocol with salt 3 as the precursor, the [CNC]-type palladium pincer complex 8 was synthesized, the solid-state structure of which was determined by X-ray diffraction analysis. Complex 8 underwent ligand substitutions with silver carboxylates producing the acetato complex 11 and trifluoroacetato complex 12. All newly synthesized pincer complexes were employed to test their catalytic activities in Mizoroki–Heck reactions. Positive mercury drop tests implied that the pincer-type complexes do not survive under the conditions of catalysis, owing to which no conclusions can be drawn regarding the catalyst variation or substrate scope.


 Please wait while we load your content...
Please wait while we load your content...